The diuranium molecule has a quadruple bond
- LPC
- Highlights
Chemical properties of actinides are not as well understood as for other elements. This is due to the fact that they are radioactive and therefore difficult to handle experimentally. In addition, theoretical studies on actinides are also challenging because of pronounced relativistic effects. PD Dr. Stefan Knecht and an international team of researchers now succeeded in calculating the most detailed electronic structure of diuranium molecule, U2, showing that it forms a quadruple bond.
In chemistry it is essential to understand the nature of chemical bonds in order to explain the reactivity of a molecule. In actinides, such as uranium, studying bonding, reactivity, or electronic structure is especially challenging because of the radioactive nature of these elements. Theoretical studies of actinides are also more complex than of lighter elements because of relativistic effects which become more pronounced the heavier an element is. Relativistic effects are the reason why some metals have special properties, e.g., gold has a yellow-red color, or mercury is liquid at room temperature. Two types of relativistic effects are distinguished. On one hand, there are scalar relativistic effects, which are caused by the very high speed of electrons and the associated increase in their relativistic mass. On the other hand, there is the so-called spin orbit interaction, which describes the influence of the charged particles (protons in the nucleus and other electrons) in the atom on the spin of an electron. These two effects change the energetics and also the spatial extent of the atomic orbitals in uranium significantly (see Figure below). The frontier orbitals 5f, 6d, and 7s of two uranium atoms are energetically closer because of relativistic effects and the latter also allow them to spatially overlap better in diuranium U2.
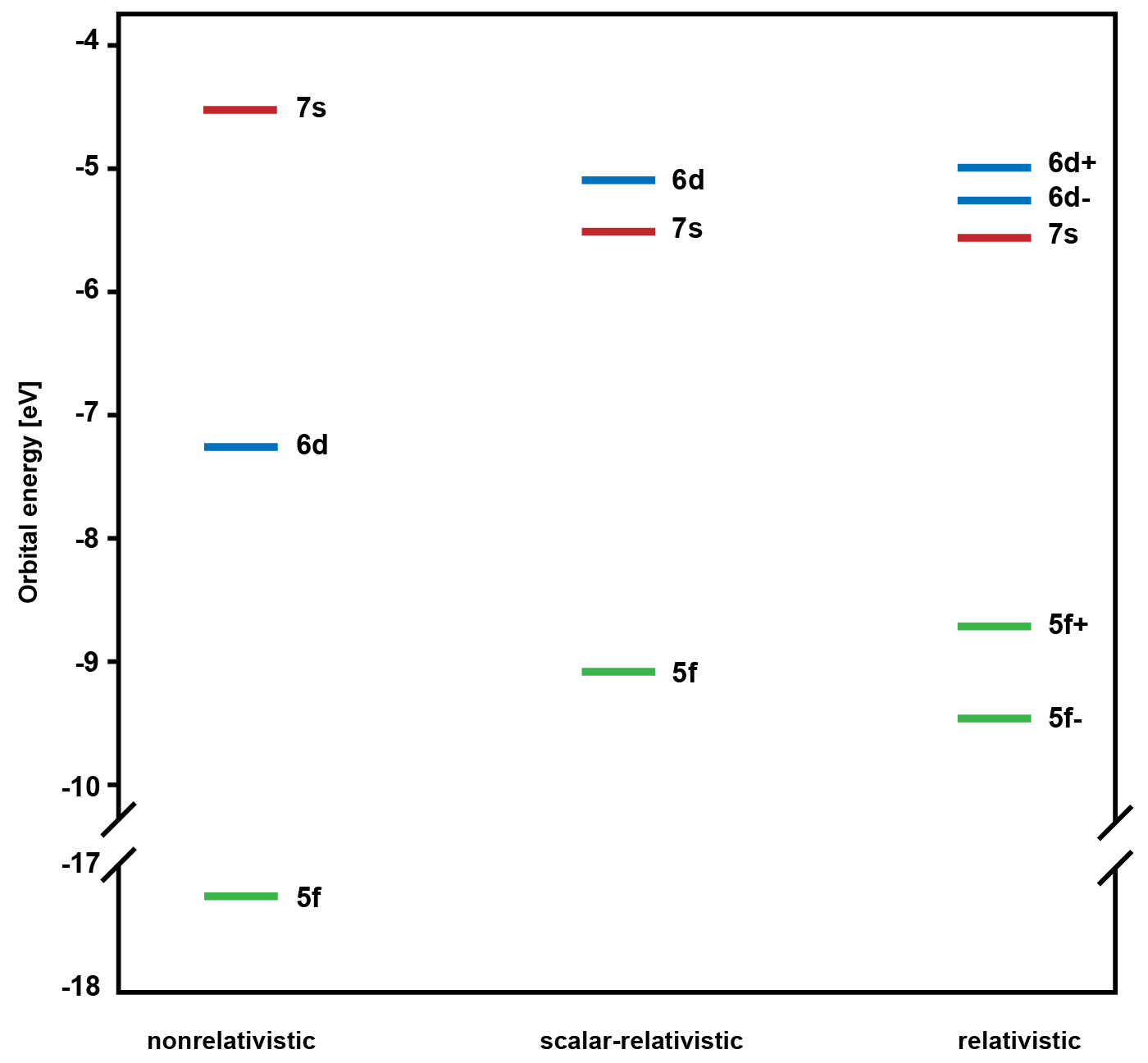
Previous theoretical studies on U2 only considered scalar-relativistic effects resulting in an effective bond order (EBO) of 4.2. The multiplicity of a bond is a value which is determined as the lowest integer value larger than the EBO itself. For diuranium thus a quintuple bond was proposed. PD Dr. Stefan Knecht and a team of international collaborators could now for the first time calculate the electronic structure of the diuranium molecule by applying quantum chemical methods considering all relativistic effects including the spin-orbit interaction in a variational approach. This revealed a different electronic ground state of U2 and an EBO of 3.8 that can be effectively translated into a quadruple bond.
In their study, they describe one of the most complex diatomic electronic structures to date, taken all relativistic effects into consideration. “Using this method, we now do not only understand the electronic structure of diuranium in unprecedented detail, these findings can also facilitate computational simulations of other heavy elements which are otherwise hard to study because of their radiative nature.” Stefan Knecht says.
Reference
Stefan Knecht1, Hans Jørgen Aa. Jensen2, and Trond Saue3,4
Relativistic Quantum Chemical Calculations Show that the Uranium Molecule U2 Has a Quadruple Bond.
Nature Chemistry 2018. DOI: external page 10.1038/s41557-018-0158-9
1) Laboratorium für Physikalische Chemie, ETH Zurich
2) Department of Physics, Chemistry and Phannacy, University of Southern Denmark
3) Laboratoire de Chimie et Physique Quantiques, IRSAMC, Université Paul Sabatier Toulouse
4) Centre for Advanced Study at the Norwegian Academy of Science and Letters, Drammensveien