
A recipe for constructing and recycling plastics
Plastics or artificial polymers have become an indispensable part of our everyday lives, and yet they cause big problems today. Athina Anastasaki and her team have discovered how polymer construction can be precisely controlled and how they can be recycled efficiently - findings that are also important for industry. For this, Athina Anastasaki (D-MATL) will receive the Ruzicka Prize 2022 on December 19. Here she provides insights into the exciting field of polymer chemistry.
"Did you know that plastic bags were invented to save the planet? I often tell the story to my students," Assistant Professor Athina Anastasaki remarks, sitting behind her desk, "it is said that Sten Gustaf Thulin invented the lightweight, long-lasting bags to prevent more trees from being cut down for paper bags. His idea was to reuse the bags." However, as Anastasaki points out, they were so cheap and quick to produce that they became a throwaway product. That’s part of our plastic problem today.
"Often there is a discovery with great potential, but then we are not being taught to use it properly," the 34-year-old Greek sums up with her straightforward manner, "On the other hand, there won‘t be a future without plastics, without artificial polymers. They can be found in almost all everyday products, from cars to toothbrushes." Now it's about finding a balance and developing solutions on how we can construct polymers in a more targeted way as well as deconstruct and efficiently recycle them, "that's my job, that's what we're investigating," Anastasaki explains.

Get to know the "ingredients" and question methods
Plastics consist of "artificial" polymers, long chains of molecules. These are assembled from molecular building blocks (monomers). However, the production of plastics with certain properties – e.g. in terms of their formability – requires polymers of different lengths and uniformity. How uniform these chains are is measured by the dispersity which is decisive for the properties of a material. Therefore, it is important to be able to control it. For a long time, however, it was not known how, which was also due to a misconception about the methods used so far.
"Most polymers are produced via free radical polymerization," Anastasaki explains. "This creates a mixture of non-uniform polymer chains of different lengths in an uncontrolled manner; this mixture has a high dispersity. The method has its advantages but is limited by the fact that the chains have "dead" ends, which cannot be changed." This way it is not possible to make precisely engineered polymers. An important advance has been achieved by the so-called controlled radical polymerization: this method produces a more uniform polymer mixture (low dispersity), with "living" chain ends that can still be modified.
In order to produce specific materials, however, you need both high and low dispersities. "There was a misconception though: free radical polymerization has always been associated with high dispersity and dead ends, controlled radical polymerization in turn with low dispersity with living ends – that's what the literature said and I often still teach these criteria to the bachelor students. But in the master's, I explain that we were able to revise that – I'm particularly proud of that discovery."
Learn how to control the construction of polymers
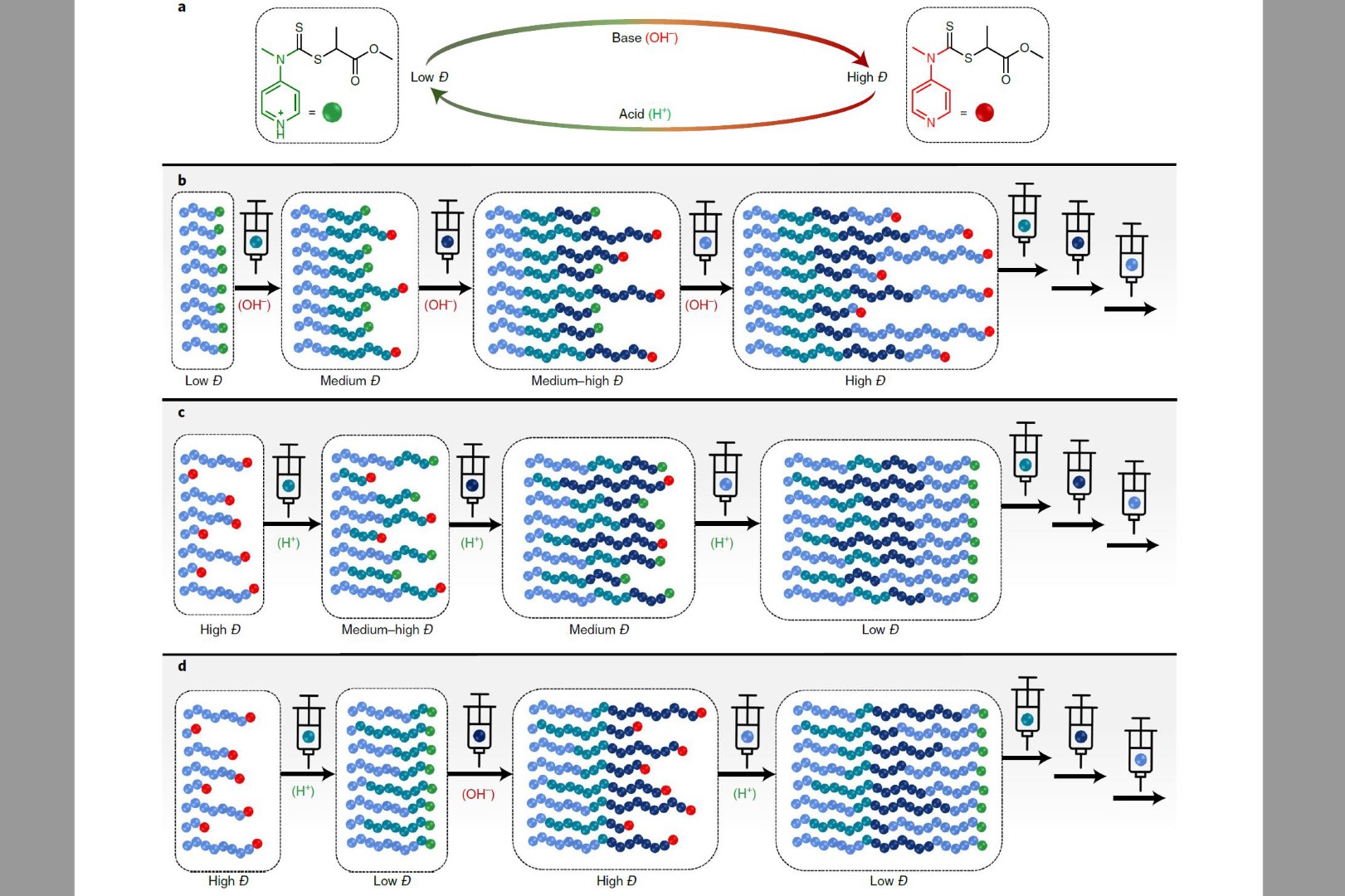
Athina Anastasaki and her team succeeded in fully controlling the dispersity of polymer materials: They found ways to increase dispersity in the fashion of free radical polymerization but without sacrificing the livingness of the ends – this was achieved by regulating the catalyst concentration or by simultaneously introducing two agents with different reactivities. In addition, Anastasaki used the chemistry of a switchable RAFT (Reversible addition−fragmentation chain-transfer) agent to control both dispersity and sequence in the synthesis of highly complex multiblock copolymers, so blocks of polymers from two or more monomer species.
"In principle, we change the reactivity of the RAFT agent, and we do that by adding acids or bases. It acts like a switch: if the RAFT agent is protonated, you get a low dispersion, without protonation you get a high dispersion – but it only changes how fast or slow the polymer chains grow. That means it doesn't affect the livingness of the polymer ends, opening up new possibilities."
Find industrially viable solutions and learn to recycle polymers
However, the switchable RAFT agents are relatively expensive – one gram is equivalent to about CHF 100 – and therefore not yet attractive to industry. In addition, polymer chemistry needs to move towards a more sustainable circular economy, which leads to another important discovery by Professor Anastasaki: she and her team developed a method for deconstructing polymers and were able to regenerate the starting monomers of Plexiglass (polymethyl methacrylate) up to a remarkable 90% and the expensive RAFT agent up to 55% without any loss of quality. Once 100 percent yield is achieved, cost-saving chemical recycling could be possible. In addition, one can change the original polymer with this method, and even create a new material.
"We took advantage of thermodynamics. If you heat a material and manage to have an active chain end, a radical so to speak, there is a chance that a reaction will be reversed" explains Anastasaki. "The problem has been that in common free radical polymerization, the end of the polymers is normally not active, so it's difficult to cleave it. To achieve that, industry currently needs to heat it up to 400 degrees, which is not very energy-friendly. Moreover, you destroy part of the monomers that you want to regenerate. We discovered that we can cleave active chain ends at much lower temperatures – at 120 degrees – which is a significant improvement."

Strive for broad application & pass on your findings
Athina Anastasaki has now received the Ruzicka Prize for these findings. "I am very honored and grateful because this prize is dedicated to the very inspiring scientist Leopold Ruzicka. Moreover, it is a challenge to receive a chemistry prize: someone had to recognize our discoveries across the whole chemistry field, not just across polymer chemistry. This is a great honor."
In the future, Athina Anastasaki would like to expand her research on depolymerization to other types of materials. Furthermore, she hopes to widen the breadth of her research on dispersity and sequence-control to applications such as mRNA, which could be better stabilized by special polymer shells. With her fundamental studies, she hopes to show the industry new possibilities, and provide her students with better insights into polymer chemistry. "My job as a researcher and teacher is also to help my students to challenge me and the textbooks," Anastasaki says, who in 2021 was awarded the Golden Owl for excellent teaching.
"As a doctoral student, I threw one of my first discoveries in the bin several times because I thought the results were not usable. In the end, it turned out it was a discovery which led to a publication. So, I encourage students to always observe carefully and discuss their observations. This is how progress and evolution happens." In the end, motivation should be fueled not only to make future discoveries, but also to learn how to use existing discoveries sustainably, so that mistakes like the one we made with the plastic bag are not repeated.
Athina Anastasaki (*1988) is from Crete, Greece. It was the extraordinary commitment of her teachers that got her interested in polymer chemistry. As an undergraduate in Athens, she published a first research paper with Prof. Marinos Pitsikalis and then traded the sunny climes of Greece for the capricious weather of Warwick, England, where she completed her PhD with Prof. Dave Haddleton in 2014 with the Jon Weaver Award for the best PhD thesis in polymer chemistry. She was awarded an Elings Fellowship, followed by a Global Marie Curie Fellowship to conduct research with Prof. Craig Hawker at the University of California, Santa Barbara. Since January 2019, Athina Anastasaki has been an assistant professor at the Department of Materials at ETH Zurich.
Ruzicka Prize Ceremony 2022
Prof. Dr. Athina Anastasaki
Constructing and Deconstructing Polymers made by Controlled Radical Polymerization
December 2022, 5– 6 pm, HCI, Lecture Hall G3, Hönggerberg
Welcome – Prof. Dr. Erick M. Carreira (D-CHAB, ETH Zürich)
Laudation – Prof. Dr. Nicola Spaldin (D-MATL, ETH Zürich)
Lecture – Prof. Dr. Athina Anastasaki (D-MATL, ETH Zürich)
Award Ceremony – Dr. Olivier Haefliger (Firmenich)
The Ruzicka Prize 2022 is sponsored by Firmenich.

Further information
Antonopoulou, MN., Whitfield, R., Truong, N.P. et al. Concurrent control over sequence and dispersity in multiblock copolymers. Nat. Chem. 14, 304–312 (2022). external page https://doi.org/10.1038/s41557-021-00818-8
Hyun Suk Wang, Nghia P. Truong, Zhipeng Pei, Michelle L. Coote, and Athina Anastasaki. Reversing RAFT Polymerization: Near-Quantitative Monomer Generation Via a Catalyst-Free Depolymerization Approach. Journal of the American Chemical Society 2022 144 (10), 4678-4684 external page doi: 10.1021/jacs.2c00963
Growing polymers with different lengths
Breaking down plastic into its constituent parts