How to build usable natural substances
Karl-Heinz Altmann learned his craft as a synthetic organic chemist at university, refined it in industry, and perfected it in academia. To this day, he replicates chemicals using blueprints provided by nature, thus trying to create the basis for new drugs and medicinal substances. After 19 years at ETH Zurich, he has retired and is now reflecting on fortunate detours, marine treasures, and the challenges of absolute freedom.

"What I do when I’m not in the lab?" Karl-Heinz Altmann laughs in surprise. "Well, I’m a football fan, and sometimes I travel with my wife and children or go to the mountains, but apart from that, I've never had time for real hobbies," he muses and does not seem at all dissatisfied. Karl-Heinz Altmann, originally an organic chemist in industry, since 2003 a Professor of Pharmaceutical Biology in D-CHAB, twice head of department, director of studies, and now emeritus, has made his passion his profession: research – preferably on the synthesis of natural substances against cancer, tuberculosis, and other scourges of humanity. "But it was a convoluted path to get here," he admits.
The way to natural product chemistry
Altmann's career began with peptide chemistry. "It kind of haunted me," he recalls with a laugh. "As postdoc, I was still working on peptides, although I had decided for general organic synthesis. But in those days, you got your jobs in a different way. My doctoral advisor had sent me to Cornell to do peptide chemistry – together with my wife, that was my condition. She is also a chemist." The plan was an academic career. Soon, Altmann got an offer from his doctoral supervisor to do his habilitation in Berlin. "Unfortunately, this did not happen. My supervisor moved to Lausanne and the offer was gone. That threw me off track for some time," Altmann admits.
The couple eventually followed the professor to Lausanne, but finally ended up interviewing for jobs at Ciba-Geigy, Roche and Sandoz in Basel, "where I was mostly considered as somebody who could do peptide chemistry, but not much else," Altmann states. There was one offer from the Ciba-Geigy though that opened the possibility to do organic synthesis. The path to natural product synthesis was paved by a happy coincidence: in 1996, Altmann turned down the offer to become section head at Ciba-Geigy Central Research. "I simply didn't feel ready for it, yet I struggled with my decision," he says. "Two weeks later, however, Ciba merged with Sandoz to form Novartis, and since I was not in a leading position with responsibility for a large group of people, I was free to move to Novartis Oncology and spend the next four years running natural products projects as a project leader."

The step into academia
Karl-Heinz Altmann remained in natural products research even after becoming a Novartis Senior Chemistry Expert. This job brought him closer to ETH Zurich: "I was a member of an ETH search committee and so was my future colleague Dario Neri. He approached me after one of the meetings. He had checked my publications and asked if I might not be interested to come to ETH. I said "yes", but seriously, at that time I did not believe that this would really lead to my application and appointment as Professor of Pharmaceutical Biology," Altmann remarks with a laugh. Ultimately, he says, the prospect of being able to research freely and teach motivated him to take the step, although the decision to leave Novartis was not easy.
At ETH, his group has been working on the total synthesis of natural products with antitumor or antibacterial activity, mycolactones and Buruli ulcer, as well as research on membrane transporters. "But most important is the total synthesis of natural products or natural product analogs and the structure-activity studies of natural products that have an effect on cancer cell growth or on Mycobacterium tuberculosis," Altmann explains.

Total synthesis of natural products
Classically, total synthesis is the synthesis of a natural substance from simple starting materials, in other words complex compounds formed from simple starting materials or building blocks. "Our work in total synthesis has always focused on natural products with promising biological activity. A complex molecular architecture alone, although fascinating and beautiful, was not enough for me," Altmann says.
"However, that a complex natural product is synthesized to be marketed directly as a drug is rare. Production from natural sources can often be so well optimized that synthesis is not needed. In addition, natural products often are not ideal drug candidates because they may not be stable, poorly soluble, or toxic," he explains. "This is why our work also puts strong emphasis on the synthesis of natural product analogs that are more readily accessible – of course, these analogs must still have the desired pharmacological and biopharmaceutical properties."
Marine sponges against cancer
Altmann's group has also ventured into natural products from marine organisms, such as sponges. Such substances often occur in the producing organism only in small quantities or the producing organism can only be harvested at a great expense. "For example, for discodermolide from the sponge Discodermia dissoluta, you have to dive about 200 meters by submarine," Altmann says, "under such circumstances total synthesis even of complex natural products can become a real option for drug development, at least in indication areas with a high profit margin, such as oncology."
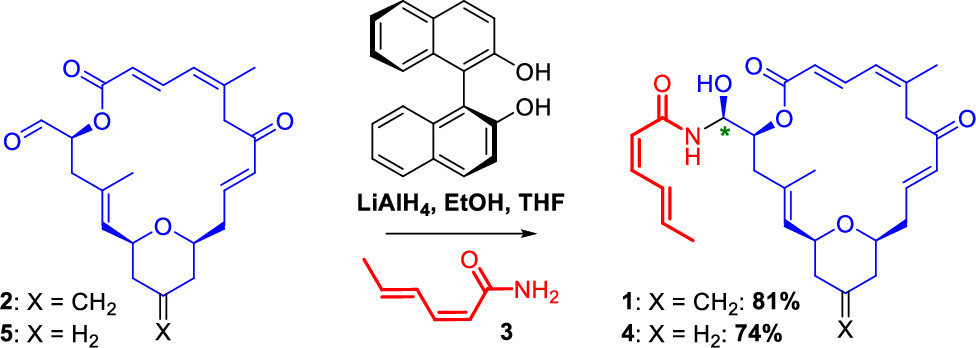
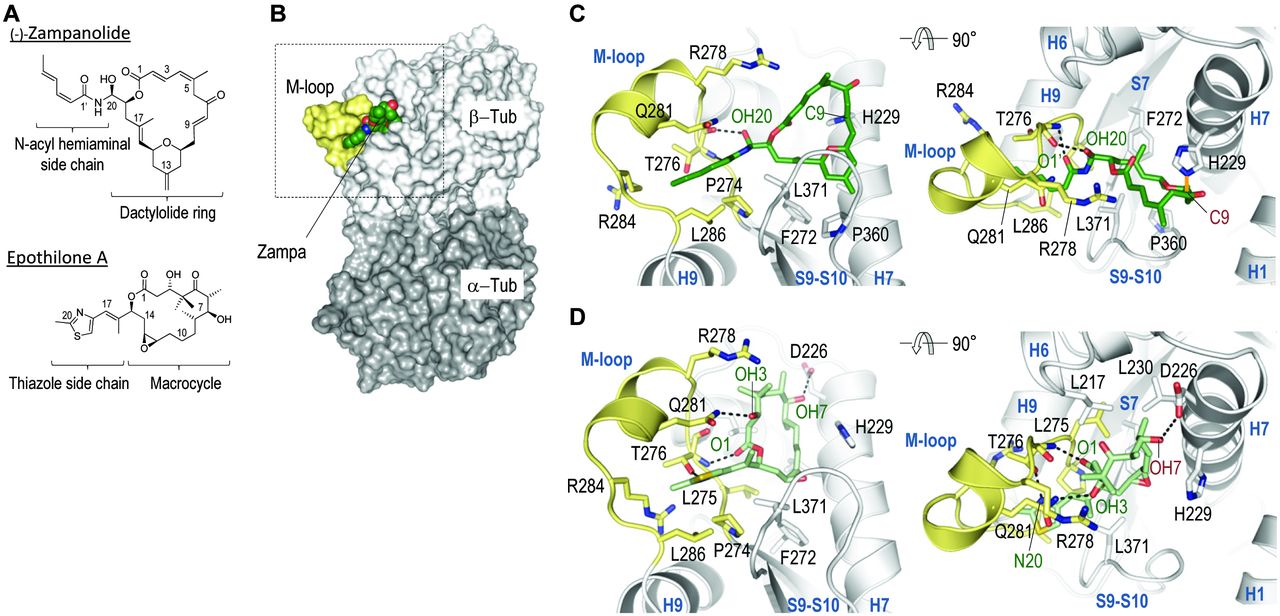
With regard to his work on marine natural products, Karl-Heinz Altmann is particularly proud of the research on the tubulin inhibitor zampanolide, a marine natural product extracted from the sponge Fasciospongia rimosa. "Our total synthesis of zampanolide has helped to clarify important questions on the fundamental biochemistry, cell biology, and structural biology of microtubules. A collaboration with a group at PSI, for instance, has led to the very first high resolution X-ray structure of tubulin in complex with a microtubule-stabilizing agent," Altmann explains.
Tubulin inhibitors are an important class of anticancer drugs, with Taxol® as the prime example, but they are also fraught with significant side effects. Therefore, current drug discovery research on tubulin-interacting agents is largely focused on their coupling to cancer specific antibodies. "But our research forms the basis for the discovery of new drugs, we are not drug developers", Altmann points out, "If you want to develop drugs all the way to the market, you are better off in industry."
Great freedom, great challenge
Has he ever regretted taking the step into academia? Never, Altmann says, although it required some adjustment. "As a professor, you have so much more freedom at all levels. I had to get used to that. At the beginning, for example, I always thought I had to report to the head of the institute if I wasn't there for a day – I didn't have to, of course. Instead, I had to learn that organizing external funds was now an important responsibility and that those funds would not be automatically renewed when the grant had expired. That was a strange feeling."
Beyond his own research and teaching, over the years, Altmann has served the Department in various other roles: first as the director of studies for the pharmaceutical study programs and twice as department head and director of studies for the Bachelor and Master programs in chemistry. As the director of studies for the pharmaceutical programs, major challenges were a complete revision of the study regulations for the Master program in pharmaceutical sciences in 2009 and leading the accreditation of the Bachelor and Master curricula by the Swiss Accreditation Council in 2011 - "an exciting but demanding process," Altmann recalls.
The same goes for his time as head of the department, which he remembers as highly interesting and rewarding, but also very stressful. Altmann's tenure coincided with an administrative investigation and ETH global budget issues, but "this was more than compensated by the opportunity to be involved in the hiring of many talented young colleagues, thereby helping to build a strong foundation for the Department's future," he states.
Prof. Karl Heinz Altmann has been emeritus since last summer but remains true to his passion for research: he is an advisor on scientific committees, and also a trusted intermediary of ETH Zurich. As such, he will be committed to ensuring scientific integrity until 2026.

“"ETH Zurich is a great university with great conditions for top research – even if I would like to see more focus on the core tasks of teaching and research and less bureaucracy. Sure, we're complaining at a high level here, but we have to be careful not to pat ourselves on the back too much or we will not keep up."”Karl-Heinz Altmann
Further Information
C.P. Bold; D. Lucena-Agell, M. A. Oliva, María Ángela; Díaz, José Fernando; K.-H. Altmann. Synthesis and Biological Evaluation of C(13)/C(13')-Bis(desmethyl)disorazole Z, Angew. Chem. Int. Ed. 2022: external page https://doi.org/10.1002/anie.202212190
T. Brütsch; S. Berardozzi, M. L. Rothe; M. R. Horcajo, J. F. Diaz; K.-H. Altmann, Org. Lett. 2020, 22, 8345-8348. external page https://doi.org/10.1021/acs.orglett.0c02974
Glaus, F.; Dedic, D.; Tare, P.; Nagaraja, V.; Rodrigues, L.; Ainsa, J. A.; Kunze, J.; Schneider, G.; Hartkoorn, R. C.; Cole, S. T.; Altmann, K.-H. Total Synthesis of Ripostatin B and Structure-Activity Relationship Studies on Ripostatin Analogs. J. Org. Chem. 2018, 83, 7150-7172. external page https://doi.org/10.1021/acs.joc.8b00193
Prota, A. E.; Bargsten, K.; Zurwerra, D.; Field, J. J.; Díaz, J. F.; Altmann, K.-H.; Steinmetz, M. O. Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer Agents. Science 2013, 339, 587-590. external page doi: 10.1126/science.1230582