
Catching the (viral) burglar
Viruses are the most ingenious burglars of the world as they manage to enter their host with permission. However, all work differently. What is a common denominator among viruses and where are their cellular entry points that could be targets for future therapies? Yohei Yamauchi, new Professor of Molecular Medicine at the Institute of Pharmaceutical Sciences, wants to find answers using cell biology, virology, and biochemistry. He is hot on the heels of influenza- and coronaviruses.
Imagine you have a satellite view of a house break-in. You can see the burglar disappear into the house, but how exactly he broke into the house is difficult to visualize. To a researcher, observing a virus enter a cell is similar. "The virus, the burglar in our story, knows the cell perfectly well, every corridor, every switch – things we may not know completely yet," Yohei Yamauchi, Professor of Molecular Medicine at the IPW and a visual thinker, explains. "In our research group, we are figuring out how viruses get into cells at the molecular level. By investigating virus behavior, we learn more about how the infectious particles enter cells, and also gain novel insights about host cell biology."
This sounds simpler than it is because every virus is unique and follows its own rules. During the cell entry process, each virus family elicits and responds to a different set of complex molecular interactions within the cell. "Therein, apart from the sheer tininess of the particle, lies the great challenge. Switching viruses from the herpes virus for my Ph.D. to the influenza virus for my postdoc took me about two years to acclimatize. The burglary strategy is so different. Hence, I won’t be surprised if a herpes virologist and an influenza virologist have difficulty understanding each other! Changing your virus of research is like moving to a different country and adopting a new culture. The common denominator is that all viruses know how to get in and get out of their host cell."

Influenza virus as a culture shocker
Yamauchi grew up in Japan, the USA, and the UK, and studied medicine at Nagoya University in Japan. He is familiar with adapting to foreign cultures – something he may have in common with viruses, as he observes with a laugh: "Viruses have to adapt to their host environment – a key aspect of my life, as well." Whilst knowing he was going to be a scientist (having published first-author papers in international journals as an undergrad), he worked in Nagoya as a medical doctor for five years to deepen his experience on human disease conditions. As a doctoral student, Yamauchi investigated mechanisms of herpes simplex virus entry: "This led me to apply as a postdoc to Ari Helenius at ETH Zurich, who was well known for his work on cell entry of influenza and other viruses."
Influenza viruses are of great medical importance. They constantly mutate which necessitates that vaccines and therapies must be continuously adapted. The influenza virus contains a single-stranded RNA genome divided into eight viral ribonucleoproteins (vRNPs). These vRNPs are protected in a proteinaceous capsid (shell) made of a regular array of viral matrix protein M1 and surrounded by an envelope derived from the host cell plasma membrane. "The defining viral action happens during cell entry, after which the virus replicates and releases thousands of infectious particles from one cell" Yamauchi points out. During entry, the virus must dismantle the capsid and release its genetic material inside the cell.
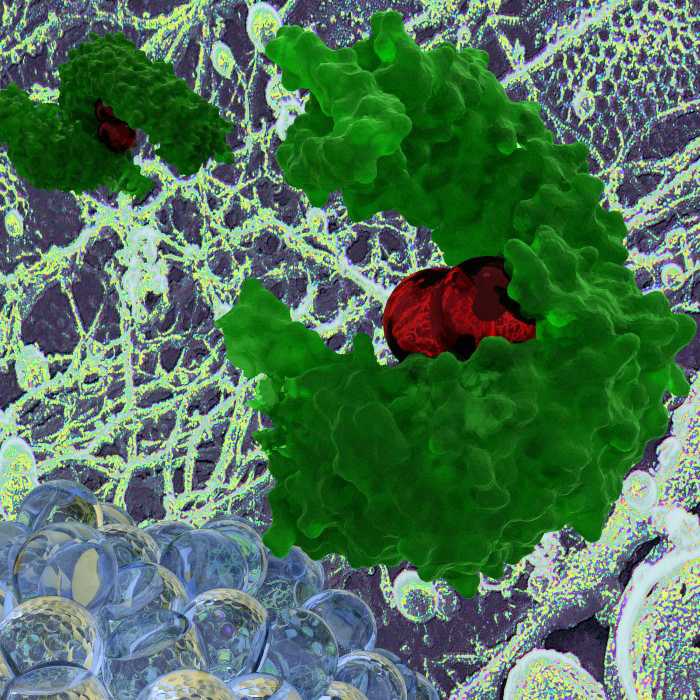
“The virus’ host appropriation is masterly. Imagine a dog in a car who would know how to push the ticket button for the parking garage and would be a good driver as well. In the case of the dog, this would seem unbelievable; but viruses can do just that and obtain the cell entry ‘ticket’. This is fascinating.”Yohei Yamauchi; picture: model of a host protein transportin-1 (green) bound to influenza A virus matrix protein)
The virus investigations of the Yamauchi group are fueled by the support of national and international interdisciplinary collaborators. As a postdoc at ETH Zurich, together with his colleagues and collaborators, Yamauchi found, for example, that the influenza virus uses the host cell's waste disposal system to break down its capsid (Banerjee et al. 2014). A protein called ubiquitin on the capsid causes the cell to classify it as "waste" and initiate disassembly via a pathway dependent on the cell’s HDAC6 protein and motility factors. This process promotes capsid breakage, release of the viral genome and infection. Yamauchi and his collaborators think HDAC6 could be a target for drug development.
"Race" in the pandemic
What about COVID-19? Yamauchi worked on SARS-CoV-2 - the causative agent of COVID-19 - while being an Associate Professor in Viral Cell Biology at the University of Bristol. "It was the most intense scientific experience in my life," he states, " we were lucky to be able to work, but I hope such times never repeat," he admits with a smile."

In September 2019, Yamauchi received an ERC Synergy grant to study ubiquitin chains in viral infections together with his collaborators. ERC allowed redirection of the funds to COVID-19 research. In 2020, Yamauchi and his team discovered a second receptor protein, neuropilin-1 (NRP1; until then ACE2 was the only known receptor), which boosts SARS-CoV-2 cell entry and spread in tissue culture and may have a role in infection of the nervous system. "Glycoproteins on the surface of viruses interact with receptors on host cells. I knew that glycoproteins with a furin protease cleavage site could utilize NRP1 for host cell entry."
In 2020, the genetic sequence of the spike protein, which is located on the surface of the coronavirus, was published, "and we noticed the presence of a furin cleavage sequence,” Yamauchi recalls. "Together with our collaborators we worked around the clock. We experimentally confirmed that NRP1 forms a complex with the spike and promoted viral entry, but we were also concerned about competition because COVID-19 was being so intensely researched." When the study was finally published in the journal Science, the EU acknowledged the work by selecting it as "project of the month", out of hundreds of ongoing EU-funded projects.

“I think we learned from the pandemic that when lives are at stake, people will work together in the most efficient ways possible to achieve a goal.”Yohei Yamauchi (the picture was drawn by his doctoral student Jiaxuan Fan)
Arousing fascination via images
Yamauchi now hopes that his personal experience will also inspire people at ETH Zurich. He has just set up his laboratory in the HCI and the team is growing. Together with his team and his collaboration partners – some of whom are in Switzerland and one of the reasons why Yamauchi wanted to return to ETH – he wants to get to the bottom of more virological mysteries and use this knowledge, for instance, to design artificial protein cages to facilitate payload delivery in cells. “Eventually, our aim is to design new antiviral strategies that contribute to medicines in the future.”
In addition, he is looking forward to teaching and to bringing his field closer to a broader audience: "I was an enthusiastic football player and striker in a league in Zurich. The fascination of the game is contagious and connects professionals and laypeople," Yamauchi illustrates. "It's basically the same with research but in virology it's trickier because people have never seen a virus. That's why I try to visualize our research with films or pictures, to help people to grasp that viruses are tiny particles possessing elegant machinery. We can gain insights from viruses about host cell biology. They are mastermind 'burglars' that act behind the scenes."

Further information
external page Yamauchi Laboratory
Banerjee et al. (2014): Influenza A virus uses the aggresome processing machinery for host cell entry Science, 346 (6208). external page DOI: 10.1126/science.1257037
Daly et al. 2020: Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science, 370 (6518). external page DOI: 10.1126/science.abd3072