
Luminous tools for living cells
Cells constantly process complex signals, be they for survival, metabolism, development, or cell death. Anyone who can spy on this internal communication has a decisive advantage regarding the development of new therapies. Michelle Frei, the new Assistant Professor of Chemical Biology and Molecular Imaging, and her team are developing chemical tools to better visualize and study cellular processes using fluorescent markers. In this article, she introduces herself.
In the third “finger” of the HCI building, Michelle Frei closes her office door and takes a seat. It feels a bit like a homecoming, doesn’t it? “To some extent,” admits the chemist, who recently returned from the USA, with a laugh.
“I'm from Switzerland and actually did my Master’s thesis with Prof. François Diederich just a few meters away from my current office. However, I associate my previous time at ETH Zurich more with my student days,” she recalls. After she finished her Master’s degree at ETH she conducted doctoral studies and a postdoc in Lausanne, Heidelberg, and San Diego. Since then, Michelle Frei has been illuminating the molecular world with new glowing compounds to better visualize cellular processes, e.g. to measure cell signals in cancer or other diseases.
Now the former ETH student is returning to the Institute of Organic Chemistry as a Professor of Chemical Biology and Molecular Imaging and wants to dig deeper into the field – a new career phase that is both a homecoming and a new beginning at the same time. “It's great that I can work in Switzerland and at ETH Zurich again. You can hardly plan such a career move in academia.”

About inspiring fireflies and useful lifetimes
Luminous compounds at a molecular level have fascinated the scientist from Aargau since she was a schoolgirl. Fireflies motivated her to take up a “Matura project” on insects during her final year in the “Kanti” (High school).
After successfully participating several times in the Chemistry Olympiad and completing her degree in chemistry at ETH Zurich, Michelle Frei soon developed chemical tools to facilitate cellular measurements. During her doctorate in the group of Prof. Kai Johnsson at EPFL and the Max Planck Institute for Medical Research, she researched fluorescent dyes for various types of microscopy – perhaps not entirely uninspired by blinking fireflies.
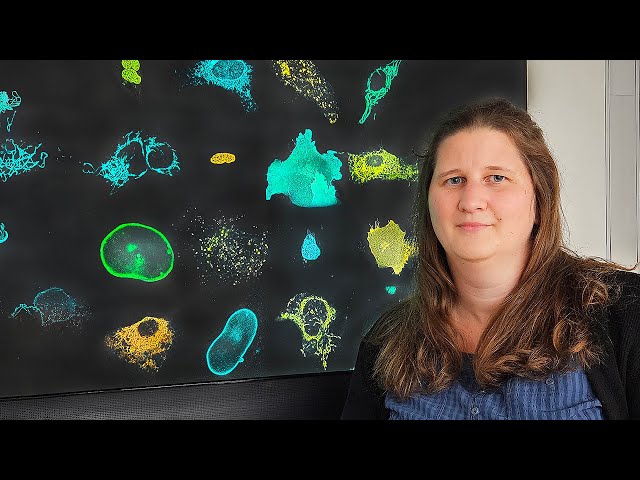
It’s important to know that fluorescent dyes (fluorophores) have certain properties: When these molecules – usually red, blue, or green dyes – are excited by light, they emit light at a specific wavelength and generate a measurable signal. This signal can be observed under the microscope, shedding light on exciting processes in tissues and cells.
However, just as the natural signals of fireflies in search for a partner differ in rhythm and length, light emission also takes place with different delays in artificial fluorophores: “The time until the dye begins to glow is called the ‘lifetime’ – a well-known, but little-noticed property of fluorophores,” Frei explains.

One fluorophore, multiple targets
The chemist recognized that fluorophores can be differentiated according to their lifetime. Based on this, in her multi-award-winning dissertation, Michelle Frei developed a new system for a so-called multiplexing approach, in which a single dye can be used to examine several targets in the cell in parallel. To do so she used the well-established HaloTag protein.
“Fluorophores are often coupled to antibodies to bring them to a specific location, but this does not work in living cells as the antibodies are too large. I prefer smaller tags – proteins that can be produced by genetically modified cells. We then introduce a fluorophore into these cells, and the fluorophore attaches itself, without any help, to the tag via a binding molecule.”
Usually, different fluorophores and tags are used for different target sites. This is challenging: not all tags work equally well, and the fluorophores also differ, for instance in how easily they enter a cell. Michelle Frei's new approach was to use only one fluorophore and produce three variants of the proven HaloTag protein – each with a different mutation that influences the lifetime and luminosity of the bound fluorophore.

“This way, the same fluorophore can be used for all tag variants. As the different tags influence the lifetime the tags fluoresce with different time delays, which we can use to distinguish them and investigate multiple targets simultaneously, e.g., the cell nucleus, the cell’s skeleton (cytoskeleton), and the mitochondria (Frei et al 2022). Until now, individual measurements dominated, and the complete picture had to be pieced together in a second step. This always left some uncertainty about how the different structures interacted with each other. Simultaneous measurements are hence better suited."
New stable and far-red tools
Moreover, as a tool developer, Michelle Frei is always driven by purpose. “As a postdoc in Prof. Jin Zhang’s lab, I researched biosensors that could be used to monitor cellular kinase activity,” she recalls. Kinases mediate many signals in the cell and regulate activities such as cell metabolism, which is also an important factor in diabetes. “We knew that we needed 4D imaging methods and ideally photostable far-red biosensors to investigate these phenomena. However, these did not exist at the time.”

Michelle Frei therefore integrated the HaloTag with a synthetic far-red fluorophore (silicon rhodamine) into a kinase biosensor and thereby created a photostable biosensor usable for these types of imaging (see Image 2). In addition, this fluorophore had the advantage that its color was orthogonal to existing sensors, “so we were able to differentiate between fluorophores that were close to each other and observe five signal pathways simultaneously” (Frei et. al 2024).
Advancing science together
Back in Switzerland, Michelle is drawn to the mountains again. Hiking is an ideal way for the scientist to switch off and relax. This allows her to recharge and gain inspiration for new projects.
At ETH Zurich, she plans to develop biosensors that do not only indicate differences (e.g. more or less signal) but also enable quantification. However, before starting with hands-on experiments she and her students need to equip and build up the lab.
That is part of the game. Michelle Frei aims to make her students well-rounded scientists: they should know their research field, be skilled in laboratory methods, and learn how to present their work at conferences. This mentoring approach was highly appreciated by her students in the USA. “It makes me very happy when my student gives a successful presentation and then excitedly reports that she has found potential collaboration partners. That's how research works. The point is not that I think for everyone, but that we advance science together.”

Michelle Frei studied chemistry at ETH Zurich and completed her doctorate with Prof. Kai Johnsson at EPF Lausanne and the Max Planck Institute for Medical Research in Heidelberg (2016-2020), where she was also a visiting scientist at EMBL. She received the EPFL Thesis Distinction and the Prix Schläfli Prize in Chemistry for her dissertation. From 2011 to 2020, Michelle Frei supported the organization of the Swiss Chemistry Olympiad. Funded by the Swiss National Science Foundation (SNSF), Frei conducted postdoctoral research with Prof. Jin Zhang at the University of California San Diego (2021-2024). She has now been working as a tenure-track Assistant Professor of Chemical Biology and Molecular Imaging at D-CHAB since 2024.
Save the date
Join Prof. Michelle Frei's inaugural lecture on April 3, 2025!
More info will follow.
Further information
Frei et al. (2022): Engineered HaloTag variants for fluorescence lifetime multiplexing. Nature Methods volume 19, pages65–70. external page https://doi.org/10.1038/s41592-021-01341-x
Frei et al. (2024): Far-red chemigenetic biosensors for multi-dimensional and super-resolved kinase activity imaging. bioRxiv preprint. external page https://doi.org/10.1101/2024.02.10.579766external page