Paradigm shift in industrial catalysis
- LAC
- Highlights
Alkene metathesis is a common chemical reaction used to produce millions of tons of propene annually. A team of researchers from the laboratories of Prof. Copéret (ETH Zürich) and Mashima (Osaka University) just discovered a new approach to activate catalysts that can reduce the standard reaction temperatures for alkene metathesis from about 400°C to 70°C. At such a low temperature even biomass-derived materials can be transformed.
Alkene metathesis is widespread in industry for many applications such as petrochemicals, polymers, or fine chemicals. Several hundreds of millions of tons of propene that are produced annually are produced by this reaction. The importance for chemical research of alkene metathesis originates in the efficiency, the generation of fewer by-products (atom economy), and the broad applicability of the reaction. The process, also known as olefin metathesis, relies on catalysts that usually consist of inexpensive silica-supported tungsten oxide (WO3/SiO2). Disadvantages of such catalysts are the high temperature needed for operation (~400°C), the low tolerance for functional groups and a low selectivity because of double bond isomerization.
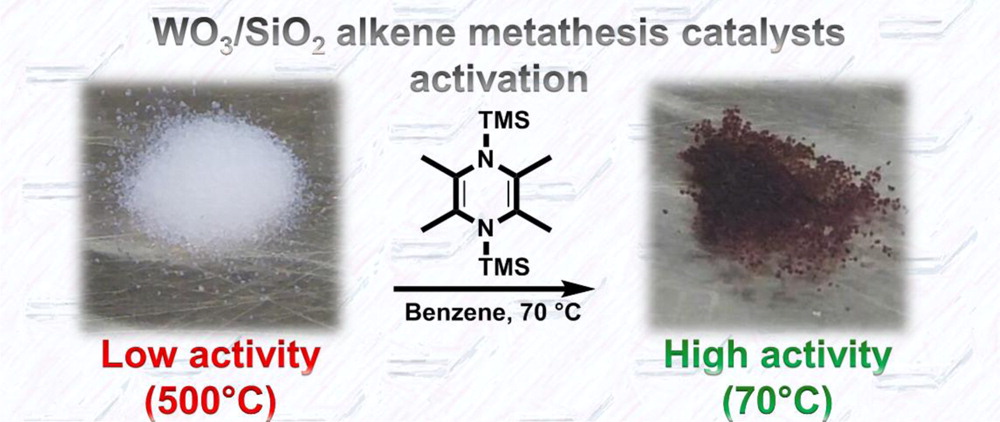
The team of researchers from the groups of Prof. Copéret and Mashima have now found a way to drastically reduce the operating temperature needed for the metathesis reaction. By treating the catalysts with an appropriate organosilicon reductant, they could observe catalysis at only 70°C. This represents not only a massive reduction in energy requirements for the metathesis reaction – it also allows for the first time to use classical industrial catalysts for conversion of a broader scope of functionalized substrates from petrochemicals, such as biomass derived compounds.
Developing a well-defined model system
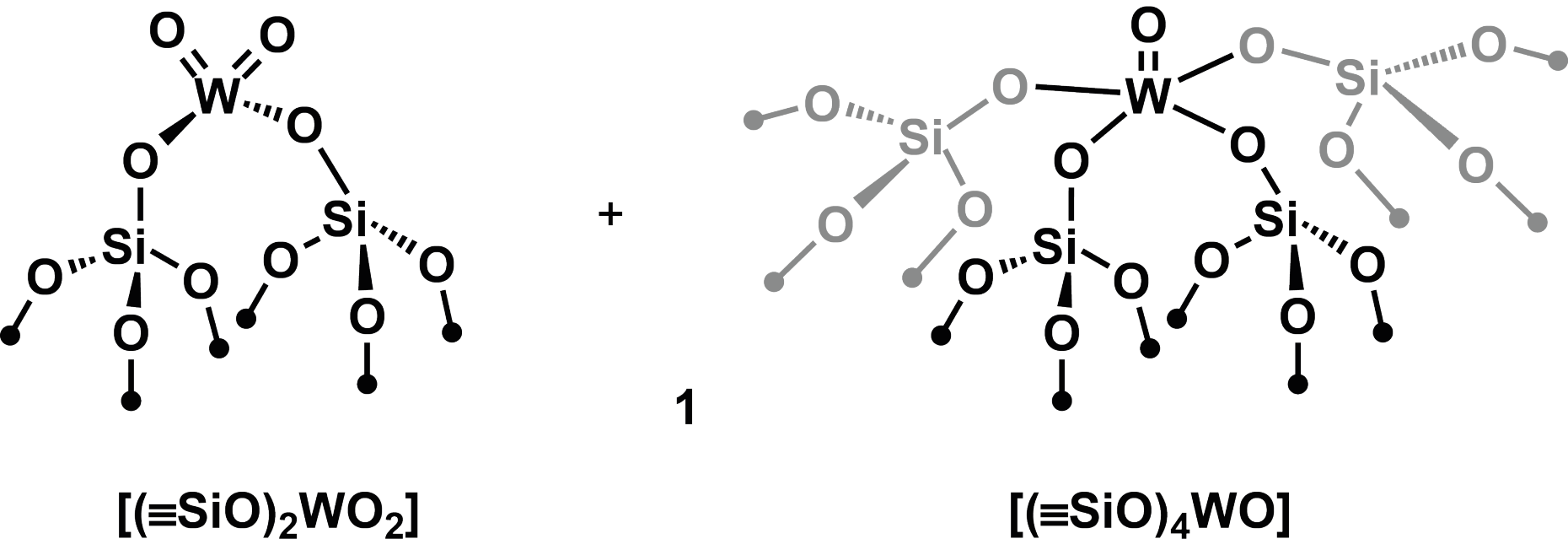
In contrast to homogenous catalysts that are well able to operate at room temperature, their heterogeneous relatives which are used in large scale in industry still require high activation and operating temperatures. In order to understand the mechanisms at the catalytic sites and to improve the activity, the first step was to produce well-defined tungsten(VI) oxo surface sites by thermolytically grafting a tungsten complex onto a silicon dioxide surface. The resulting white powder consists of a 1:1 mixture of [(≡SiO)2WO2] and [(≡SiO)4WO].
Although the metal sites of this model catalyst are well-defined, it is only active at temperatures above 400 °C and with a very low turnover frequency in this form.
Improved catalytic activity through reduction
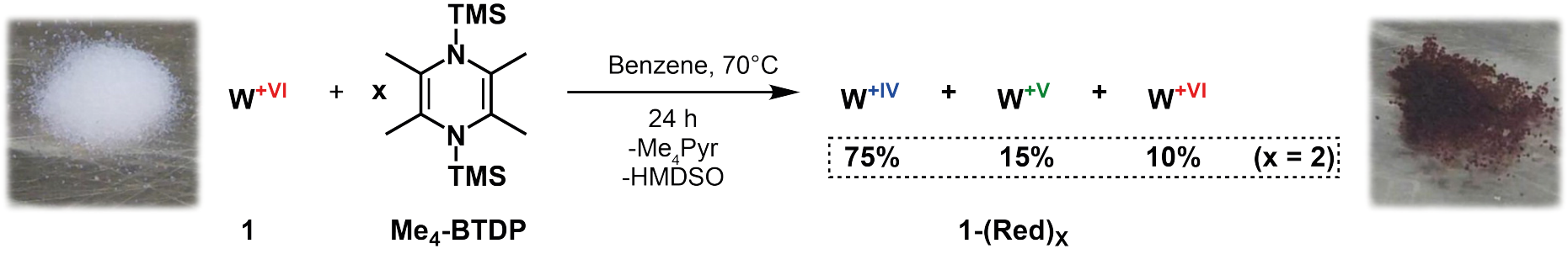
Key to overcome the high operation temperature and low activity is the reduction of the metal center. In order to avoid the formation of coproducts during reduction that can hinder the catalytic activity by staying adsorbed on the surface, the international research team used a metal- and salt-free organosilicon reagent: Reducing the well-defined catalyst with 2,3,5,6-tetramethyl-1,4-bis(trimethylsilyl)-1,4-diaza-2,5-cyclohexadiene (Me4-BTDP) resulted in a dark purple solid which showed high metathesis activity at only 70 °C. Successfully converted olefin substrates were cis-4-nonene, 1-nonene, ethyl oleate, and cyclooctene.
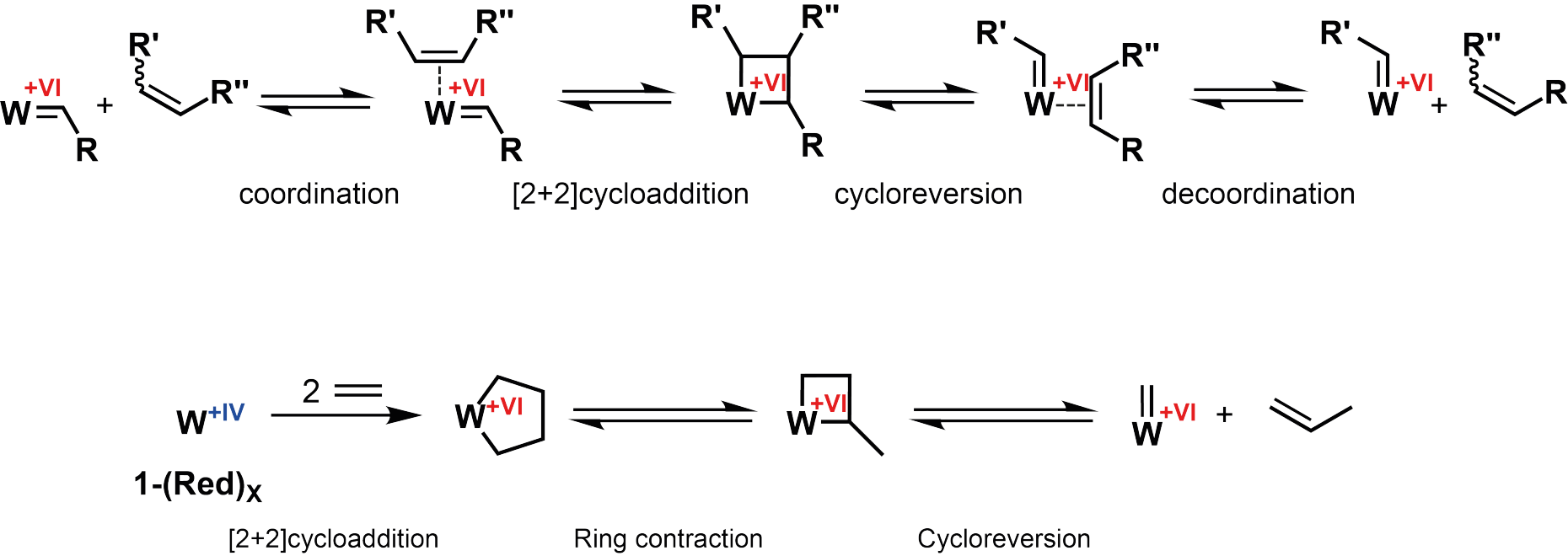
The W(IV) centers which are predominant (75% of the species) in the reduced form of the catalyst are responsible for the high activity of the alkene metathesis. The initiation mechanism is different from the one encountered with homogenous metathesis catalysts: First, a metallacyclopentane is formed which undergoes ring contraction to eventually form a W(VI) alkylidene species. The presence of such alkylidene in well-defined homogeneous catalysts is the reason for their high activity.
The proposed mechanism shows that the alkylidene species is vital for catalysis initiation. Formation of this species is the limiting factor in industrial heterogeneous (WO3/SiO2) catalysts, since harsh operating conditions such as temperatures of ca. 400 °C are required. Eventually the reduction was performed on the heterogeneous catalysts that are used in industry. The use of this reductant proved to also be efficient at 70 °C for the industrial catalysts WO3/SiO2 and MoO3/SiO2.
Keeping catalysts cool but active
By using an appropriate reducing agent, the international team researchers from the laboratory of Prof. Copéret (ETH Zürich) and Mashima (Osaka University) could enable classical heterogeneous metathesis catalysts to perform at much lower temperatures, 70 °C in place of greater than 400 °C. The reduction of the metal centers triggers the rapid generation of alkylidene species, enabling these catalysts to perform like their homogeneous, well-defined relatives. This results in improved selectivity and enables for the first time to directly use industrial catalysts for the efficient low temperature conversion of biomass-derived products via alkene metathesis processes.
Press release Osaka University external page 大阪大学 (japanese)
References
external page Low Temperature Activation of Supported Metathesis Catalysts by Organosilicon Reducing Agents: Victor Mougel, Ka-Wing Chan, Georges Siddiqi, Kento Kawakita, Haruki Nagae, Hayato Tsurugi, Kazushi Mashima, Olga Safonova, and Christophe Copéret. ACS Central Science 2016.
external page Activation of Supported Olefin Metathesis Catalysts by Organic Reductants
Inventors: Coperet Christophe, Mougel Victor, Mashima Kazushi, Tsurugi Hayato, Nagae Haruki.
European patent WO2016095061 (A1), 2016-06-23.