Highly Efficient Catalyst for Hydrogen Production from Formaldehyde
- LAC
- Highlights
In their latest publication, Prof. Grützmacher and his research group demonstrate that formaldehyde can be used as a very suitable small organic molecule to store hydrogen. Newly developed diolefin-ruthenium complexes can catalyze the production of H2 from formaldehyde and water in the presence of a base.

The need for energy storage
On May 21 2017, the Swiss people said yes to the Energy Act, accepting the Energy Strategy 2050 which implies the use of renewable forms of energy and promotion of energy efficiency. With a mandate to move away from nuclear power and our global resources of fossil fuels diminishing, there is now the need to develop new and safe ways to store and distribute energy from whatever sources it is generated. One candidate for clean fuel is hydrogen, despite it is not an energy source that can be found in nature. Hydrogen could be burned to water or used in a fuel cell to directly produce electricity, which makes it a very clean energy carrier. Hydrogen – H2 in its molecular form – is produced industrially from hydrocarbons through steam reforming but can also be produced without fossil fuels through electrolysis. This process uses electric energy to split water into O2 and H2 without CO2 as a by-product.
Molecular hydrogen, however, is not easily manageable without overcoming some storage issues. First of all, it’s highly flammable, and with a boiling point of −253 °C it has to be contained in pressure tanks, which add weight for e.g. hydrogen-driven vehicles. An option to overcome the storage and handling problems of hydrogen is storing it chemically in a more stable molecule. This chemical should optimally have a relatively high hydrogen content. One of the smallest organic molecules is formaldehyde which can be used as aqueous solution. Prof. Grützmacher’s research group now developed a new and highly performant catalyst system that can release hydrogen fast from aqueous formaldehyde under very mild conditions [1]. This makes formaldehyde an attractive H2 storage system with a high weight percentage of hydrogen.
A catalyst with remarkable features
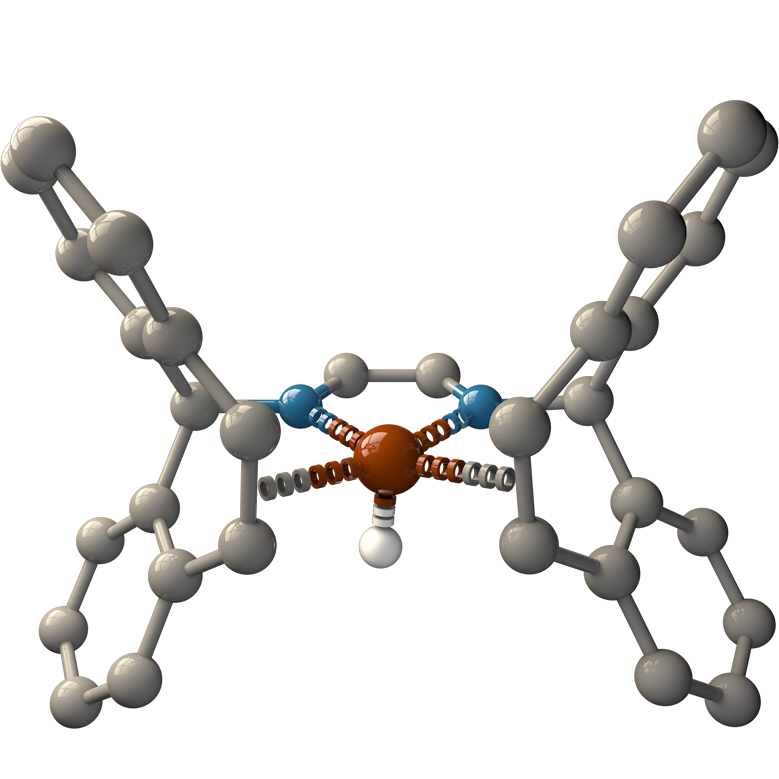
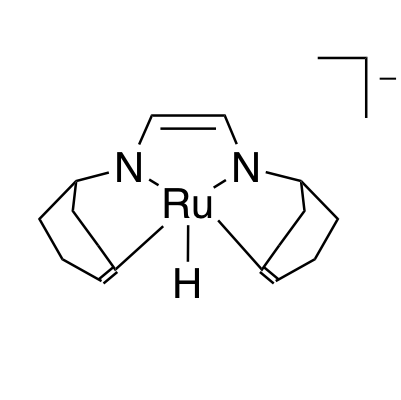
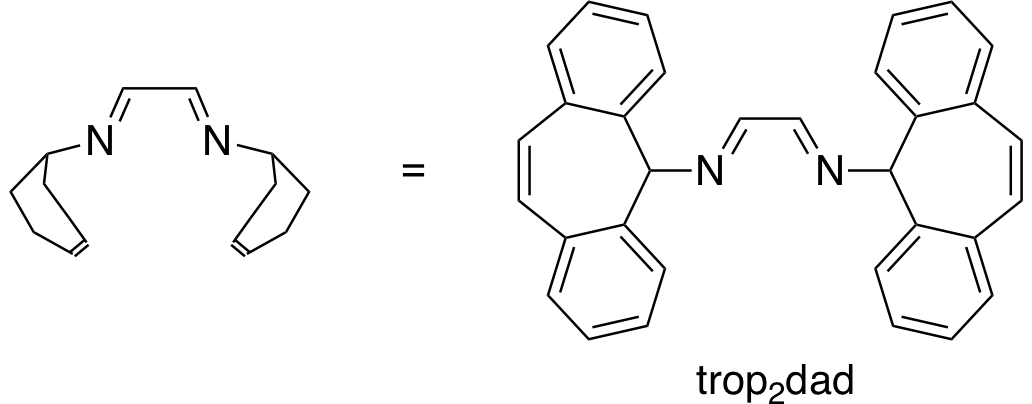
The catalysts that are able to produce H2 from aqueous formaldehyde solutions are developed by Dr. Monica Trincado, Oberassistent in Prof. Grützmacher’s group, and her co-workers. Consisting of ruthenium metal complexes, all catalysts have a lightweight and cheap to produce organic ligand in common: trop2dad (an example of a catalyst and the ligand are shown in the figure below). Under basic conditions, the ruthenium catalysts achieve a high turnover number, (the number of times the catalyst is able to react before degradation) of up to ~30’000 and a high turnover frequency, (the speed at which the catalyst operates) of nearly ~1’800 hours–1. The catalysts produce H2 and carbonate in high yield at a very low reaction temperature of 60 °C.
In the aqueous solution of formaldehyde, called formalin, various hydrated and polymeric species of the formaldehyde molecule exist – such as methanediol and paraformaldehyde. Therefore, various reaction pathways for the conversion of formalin have to be taken into account. One described pathway even involves the production of carbon monoxide as an intermediate, a molecule that frequently “poisons” the catalysts by deactivating them completely. Yet, the ruthenium catalysts are extremely adaptable to all the mentioned conditions and even operate in a carbon monoxide atmosphere. Because of their uniqueness to adapt to these conditions, Prof. Grützmacher and Dr. Trincado call their newly developed ruthenium complexes ‘catalytic chameleons’.
Density functional theory (DFT) calculations support the proposed reaction mechanism, suggesting the main reaction pathway being the dehydrogenation of the methanediol species in solution. During the reaction, the ruthenium center switches oxidation states between zero and +II and the trop2dad ligand acts as an intermediate hydrogen storage.
Dr. Trincado points out that the catalyst could be even used to detoxify water that is polluted with formaldehyde, as has been previously suggested by others working with a related catalytic system which is efficient at very diluted concentrations. The end products would be sparkling water and non-toxic hydrogen gas.
Direct generation of electric power
His goal, Prof. Grützmacher says, is not necessarily the production of hydrogen gas, but to use the catalyst to produce electricity using so-called “metal organic fuel cells”. Such fuel cells are also part of his research, and he has already developed in collaboration with the group of F. Vizza at the CNR in Sesto Fiorentino a similar catalyst that can act directly at the electrode to generate electricity from alcohol-water mixtures [2,3]. Such a system increases energy efficiency and eliminates the disadvantages of gaseous H2. Also, he points out, the starting source for formaldehyde synthesis should be recycled CO2, and not fossil fuels.
References
[1] Trincado M., Sinha V., Rodriguez-Lugo R. E., Pribanic B., de Bruin B. & Grützmacher H. external page Homogeneously catalysed conversion of aqueous formaldehyde to H2and carbonate Nature Communications, 2017, 8, 14990
[2] Annen S., Bambagioni V., Bevilacqua M., Filippi J., Marchionni A., Oberhauser W.,Schönberg H., Vizza F., Bianchini C. & Grützmacher H. external page A Biologically Inspired Organometallic Fuel Cell (OMFC) That Converts Renewable Alcohols into Energy and Chemicals Angewandte Chemie International Edition, 2010, 49, 7229-7233
[3] Rodriguez-Lugo R. E., Trincado M., Vogt M.,Tewes F., Santiso-Quinones G. & Grützmacher H. external page A homogeneous transition metal complex for clean hydrogen production from methanol–water mixtures Nature Chemistry, 2013, 5, 342-347