New views on a 40-year-old catalyst
- D-CHAB
- LAC
- Highlights
Developed almost 40 years ago, Titanium Silicalite-1 (TS-1) has been successfully used as a catalyst to convert propylene into propylene oxide, an important commodity chemical, which is used e.g. to produce plastics like polyurethane. Now, a team of researchers at ETH Zurich, the University of Cologne, the Fritz-Haber-Institute, and BASF, revealed the actual and surprising reaction mechanism.
Propylene oxide is an important commodity in the chemical industry, and used for the production of plastics, antifreeze agents, and hydraulic fluids. More than 11 million tons of propylene oxide are produced annually, of which over 1 million tons are already produced by oxidation with hydrogen peroxide (H2O2). The chemical reaction is catalyzed by Titanium Silicalite-1 (TS-1), a porous, crystalline material that consists of silicon dioxide, and incorporates small amounts of titanium atoms (around 1 to 4 titanium atoms per 100 silicon atoms). Water is the only byproduct. Although this catalytic process has been successfully used since the development of TS-1 almost 40 years ago, the exact mechanism of catalysis was unknown. However, it was the consensus in the chemical research community that the active sites in TS-1 must be isolated titanium atoms, providing the basis for the unique reactivity of the catalyst.

A team of researchers from ETH Zurich, the University of Cologne, the Fritz-Haber-Institute in Berlin, and BASF, questioned this assumption. In a study now published in Nature, the researchers could show that two titanium atoms activate H2O2 during the catalytic process, indicating that titanium atoms are in fact not isolated, but rather cooperating entities.
Based on 17O solid-state NMR spectroscopy, the researchers could show that during oxidation with H2O2, peroxo intermediates are formed that involve two titanium atoms. By computational modelling the authors further show that these dinuclear sites are indeed highly competent for the oxidation of propylene. Interestingly, the operating mode of such dinuclear sites parallels the well-established reaction mechanism of peracids, which are some of the oldest and most prominent reagents for alkene oxidations. The study also shows that the dinuclear sites are particularly efficient in propylene epoxidation and should be considered for the development of improved catalysts.
“None of the methods that we used in this research are fundamentally new; however, many things had to happen at the same time to enable this study”, says Christophe Copéret, the corresponding author of the publication. “Our laboratory only recently developed a method to synthesize 17O-enriched hydrogen peroxide, which was one key ingredient of our recent work. In addition, our research group gained significant experience with low-temperature NMR spectroscopy over the recent years, which enabled us to conduct this study. Also Dr. Henrique Teles, BASF, and a co-author of the study, emphasizes: “If you try to optimize a catalyst on the basis of a wrong assumption, it is very difficult and can lead you in the completely wrong direction. It was therefore important to examine this assumption more closely.”
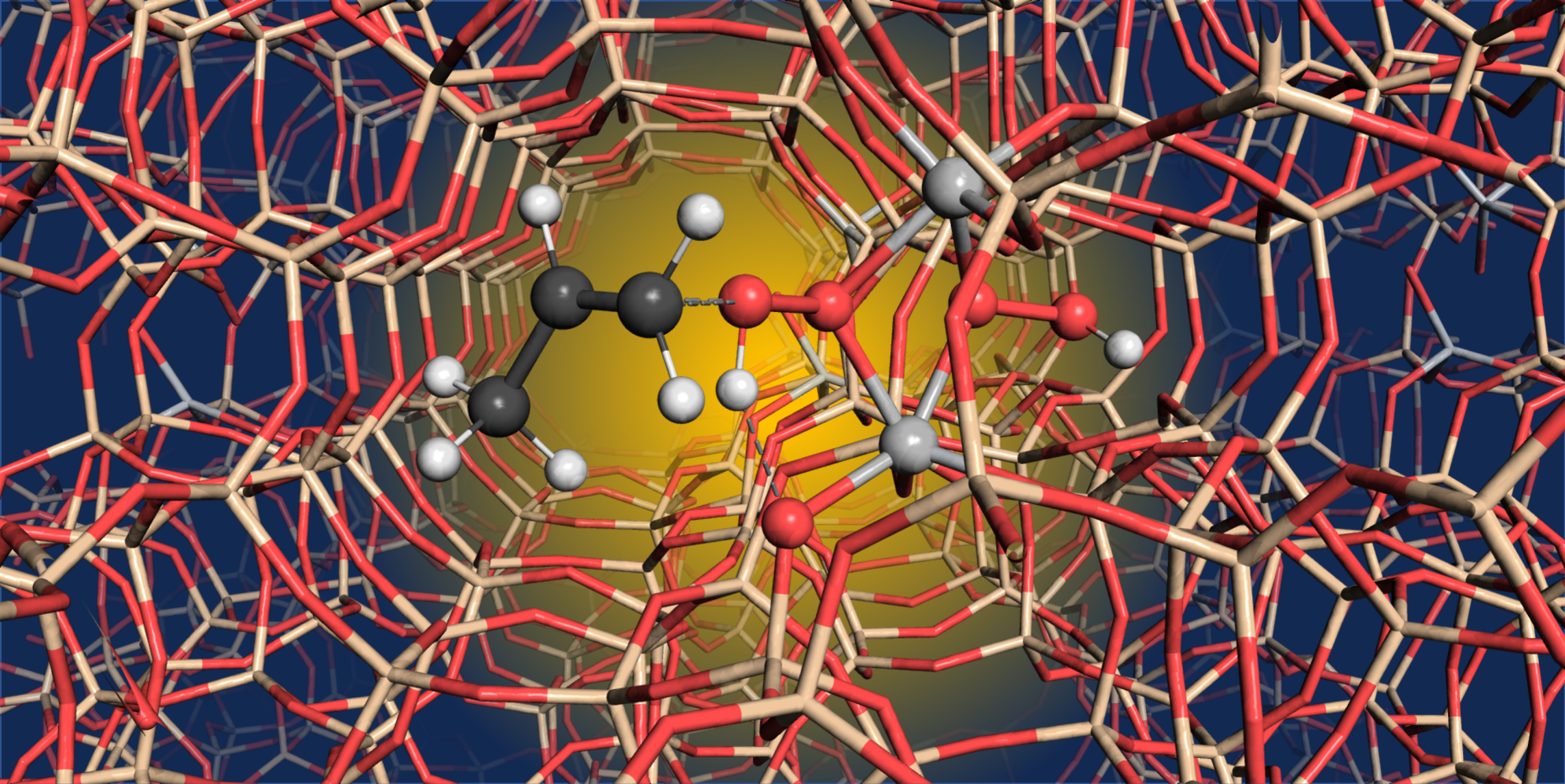
“We have worked for many years to elucidate the reaction mechanism of a homogeneous titanium catalyst and found that – contrary to the assumptions in the literature – the hydrogen peroxide is activated by a titanium pair. It was really a special moment when we saw in the current study that the findings from homogeneous catalysis also apply to heterogeneous catalysis,” said co-author Prof. Albrecht Berkessel from the University of Cologne. And Dr. Thomas Lunkenbein, co-author from the Fritz Haber Institute in Berlin, adds: “We are very pleased that we were able to make a contribution to this study. With our analytics, we were able to substantiate the conclusions. The knowledge of a diatomic active center is of fundamental importance and opens up new possibilities in catalyst research.”
The development of catalysts is often a black box, that relies on many experiments, large-scale screening, and luck. Knowing the structure of the desired active site is of utmost importance for the rational development of catalysts. Elucidating the reaction mechanism of the heterogenous catalyst TS-1 is highly surprising, and sheds new light on this 40-year catalyst, which will help in improving and developing other homogeneous and heterogeneous catalysts.
Reference
Gordon CP, Engler H, Tragl AS, Plodinec M, Lunkenbein T, Berkessel A, Teles JH, Parvulescu AN, Christophe Copéret C. Efficient epoxidation over dinuclear sites in Titanium Silicalite-1. Nature (2020) doi: external page 10.1038/s41586-020-2826-3
Further Information
Website Research Group Copéret
external page Joint Press Release
Nature News&Views article about the publication: Bert M. Weckhuysen, external page "Fresh evidence challenges the consensus view of active sites in an industrial catalyst"