Searching the missing puzzle piece
- D-CHAB
- LAC
- LPC
- LOC
- IPW
- Highlights
- ICB
Lignin is one of the most common organic compounds in the world, with an annual production of 20 billion tons. What if this complex substance could be easily converted into sustainable fuel? In 2017, Patrick Hemberger, scientist at the synchrotron of the Paul Scherrer Institute in Villigen, was the first to find an efficient way to investigate this process. For his work on reactive intermediates in heterogeneous catalysis, he is awarded the 2020 Ruzicka-Prize. Here, he introduces himself.
One can hear strange noises echoing through the hall, while standing on a scaffold, looking at the huge greyish circular path below. Constructions like this are probably one of the fastest "race tracks" in the world external page synchrotrons like the one at the Paul Scherrer Institute (PSI) in Villigen, don`t send runners onto their track, but electrons. Here, they move through the vacuum on their 288 m long circular path at almost the speed of light. More important, however, is what they emit when deflected: photons. These enable researchers like Patrick Hemberger to observe things that would otherwise not be discovered – e.g., new intermediates. These intermediates are the "track switches" in chemically complex reaction paths between the starting material and the final product, and are crucial when processes should become more economical – such as fuel production from natural materials like lignin. Provided one knows the right method.

Catch & steer instead of cook & look
"For a long time, the only approach to make a reaction more selective was cook & look: one varied the process conditions like the temperature or the pressure and then checked, if the final product is the desired one,” Hemberger explains. That method was inefficient. In order to optimize the process, he says, one has to know the reactive intermediates – molecules such as propyl radicals, for example, which are created in the course of a chemical reaction under certain conditions while being very reactive. However, a whole host of molecules are formed during the reaction when the starting materials are converted, and all of them can influence the formation of the desired product [see video below]. The aim of Patrick Hemberger's research at PSI is therefore to find and understand the reactive intermediates, and thus to control these processes. "If you understand the intermediates, you understand the chemistry behind the reactive systems – they are the missing puzzle piece in the whole reaction mechanism," emphasizes Hemberger.
Unconventional career, unconventional methods
Hemberger is originally from Würzburg, Germany. He took an unconventional path and was trained as a chemical laboratory assistant before beginning his chemistry studies in Würzburg. During his diploma thesis in Professor Ingo Fischer's group, he came into touch with synchrotron radiation. "The idea was to produce reactive intermediates in small reactors and measure their photoelectron spectrum. We wanted to see if we could predict these spectra." Hemberger continued to work in this group. In 2011, after only two and a half years, he received his doctorate on spectroscopy and dynamics of reactive intermediates. Shortly afterwards he moved to PSI in Villigen. Since then, he has been working there as a scientist on the vacuum ultraviolet beamline (VUV). He conducts his own research projects and is responsible for development and maintenance of the beamline as well as for the user support.
Being a father of two children, Hemberger likes to regain the energy for all these tasks during family outings or while riding his road bike, but the inspiration for research comes mainly from close contact with the beamline users. During his work he soon realized that intermediates are the key to understanding the chemistry in reactive systems. However, standard tools such as classical mass spectrometry or nuclear magnetic resonance have so far failed to detect these intermediates. The half-life of these molecules is quite short, Hemberger explains, "you have to make sure that they survive until you can detect them." In addition, he says, the molecules are difficult to distinguish. Some of them have the same molecular formula (and thus the same mass, given the same number and type of atoms), but their structures are different. Hemberger therefore established iPEPICO (Imaging Photoelectron Photoion Coincidence Spectroscopy) as an in-situ analysis instrument. This is a method that uses VUV synchrotron radiation and combines mass spectrometry with photoelectron spectroscopy.
A matter of combination
The molecules are ionized by VUV synchrotron radiation, which is a special light. "It removes electrons from the outermost molecular orbitals. This enables high sensitivity and selectivity," says Hemberger, "because unlike in typical mass spectroscopy, the molecules are not fragmented. We can fine tune the energy of vacuum ultraviolet light that we can fully suppress this fragmentation process." In this way, intermediates and fragmented molecules produced by the method are no longer confused with each other. However, the intermediates can still be present as isomers, which means they have different spatial arrangements. These molecules are difficult to distinguish as well. By combining mass spectrometry and photoelectron spectroscopy, the molecular mass is obtained as well as the photoelectron spectrum. "This gives us kind of a unique fingerprint of each individual molecule", Hemberger says, "thus, the peaks can be assigned to the molecules and even to isomers."
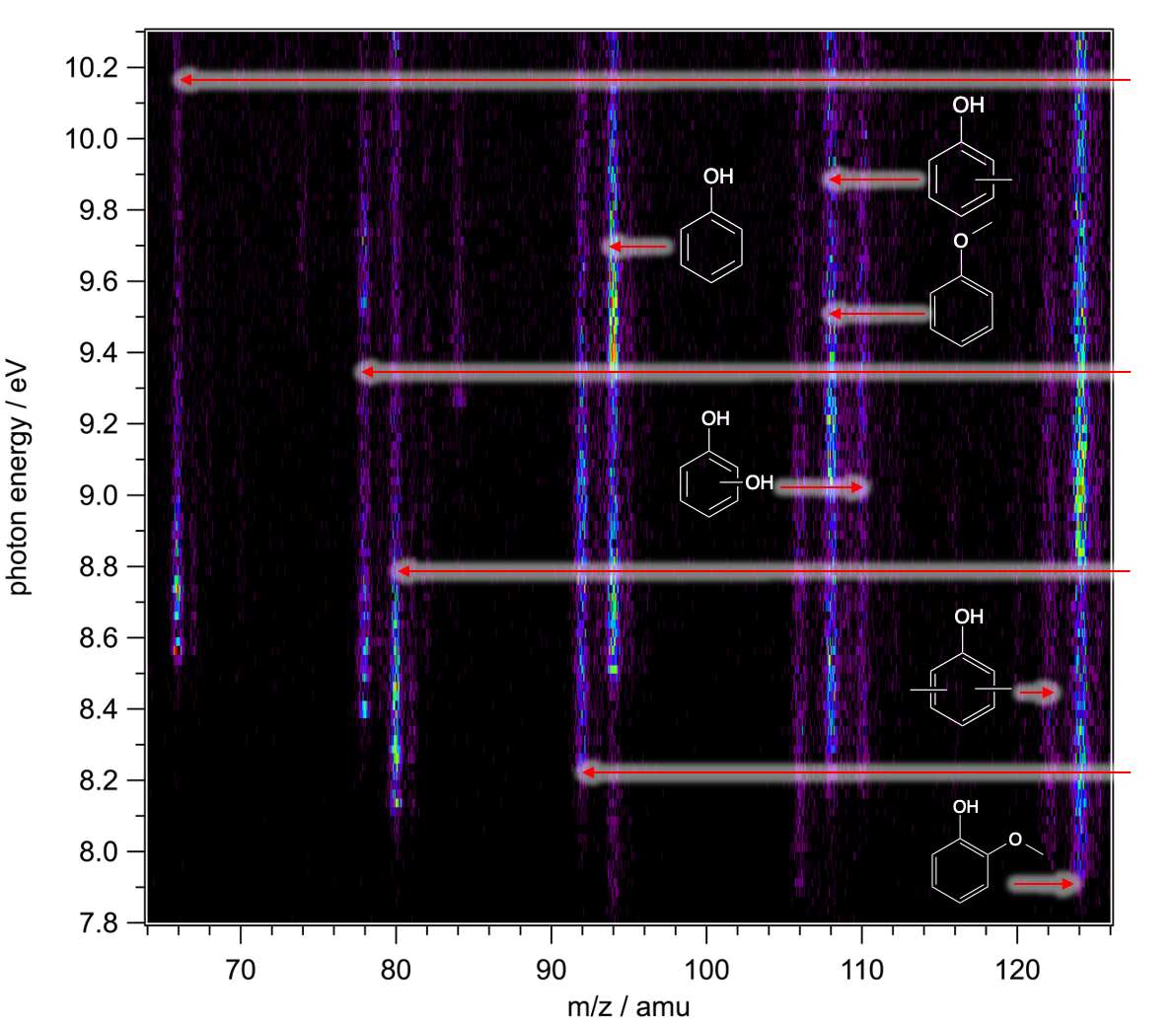
Hemberger pioneered this application and succeeded in the isomer-selective detection of highly reactive combustion intermediates or in the revelation of fulvenone as a central reactive intermediate in the catalytic pyrolysis of the lignin component guaiacol: "Lignin is a macromolecule, consisting of different phenolic units. First you need to crack it and then you can convert it on a catalyst to get the desired products," Hemberger explains. To this end, he investigated the decomposition mechanism of the organic compound guaiacol at around 400° C without oxygen (pyrolysis). The multi-porous material zeolite served as a catalyst and surface for this reaction. This could help to make the conversion of the CO2-neutral, natural material lignin to fine chemicals and fuels via catalytic fast pyrolysis more economical in the future.
For his work on the elucidation of the pyrolysis mechanism by investigating reactive intermediates, Hemberger is awarded the 2020 Ruzicka-Prize: "It is a huge honor for me, that my research is gaining such recognition, even outside PSI and my own research community". In the future he will continue to take a closer look at different catalytic phenomena, including coking. "But lignin will also keep us busy," Hemberger says with a laugh, "at least for the next three years."
Please note: Ruzicka-Prize ceremony
The ceremony takes place on November 24, 5-6 pm and can be watched via YouTube livestream.
More Information on the Ruzicka-Prize-homepage.
Further Information
external page Paul Scherrer Institute in Villigen
Hemberger, P., Custodis, V., Bodi, A. et al. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat Commun 8, 15946 (2017) external page DOI: 10.1038/ncomms15946