Speed dating with radioactive heavyweights
- D-CHAB
- LAC
- Highlights
They have bizarre names, are heavyweights on the molecular scales, are radioactive and artificial: superheavy elements or transactinoids are the exotics of the periodic table and Patrick Steinegger's only passion. At PSI and partner institutes, the radiochemist uses particle accelerators, plutonium targets and gold (among others) to track down and characterize these oddities. As an assistant professor at the LAC, he now aims to introduce the field to students at ETH Zurich.

It is a warm morning at the Paul Scherrer Institute (PSI). Not far from the proton accelerator, Patrick Steinegger opens a window and smiles while thinking about the old periodic tables of elements present in so many classrooms. "Many people think this is a closed system, but new elements and isotopes are being discovered all the time," he says as he points to a colorful map on the office wall.
"We radiochemists are interested in the radioactive ones, those that are unstable and decay easily. Such decays even exist in the human body, mainly due to potassium-40. In general, about 90 percent of all known isotopes and about one-third of all known elements are radioactive." Some of them, however, have hardly been studied because of their short lifetimes, Steinegger explains: "That is what we do: We create such elements, detect them and get to know them chemically within single seconds" – in other words, atomic high-speed dating with a very low probability of succeeding.

A lottery draw with super heavy "consequences"
Sounds like crazy research? "When I was a student in my hometown of Bern, I thought so too," Steinegger recalls with a laugh. A few years later, however, he found himself at PSI. After a lottery draw during the internship distribution, he traded for the super heavy elements. "I swapped my lot with someone. That's how I joined the group of Robert Eichler, who is my superior at PSI and an exemplary scientist." Later, after completing his Master's degree in laser spectroscopy, Steinegger returned to PSI. He earned his doctorate in the field of superheavy elements in 2015 and then worked for four years at the particle accelerator in Dubna, Russia – next door to Yuri Oganessian, namesake of the heaviest element known to date. Back in Switzerland, Steinegger became a group leader at PSI. Shortly thereafter, he was appointed to the newly established professorship of radiochemistry at ETH Zurich. Here he aims to familiarize students with the radioactive elements that are his profession, hobby and passion.
Research at the limit of possibility
These elements and their associated radioactive isotopes belong to the so-called transactinoids. These "exotics," located in period 7, groups 4 to 18 of the periodic table, have strange names like Roentgenium or Darmstadtium and are considered superheavy because they have more than 103 protons in their nucleus. "Usually, you can't find these elements in nature," Steinegger says, "they are created artificially by inducing collisions of elements with certain atomic numbers."
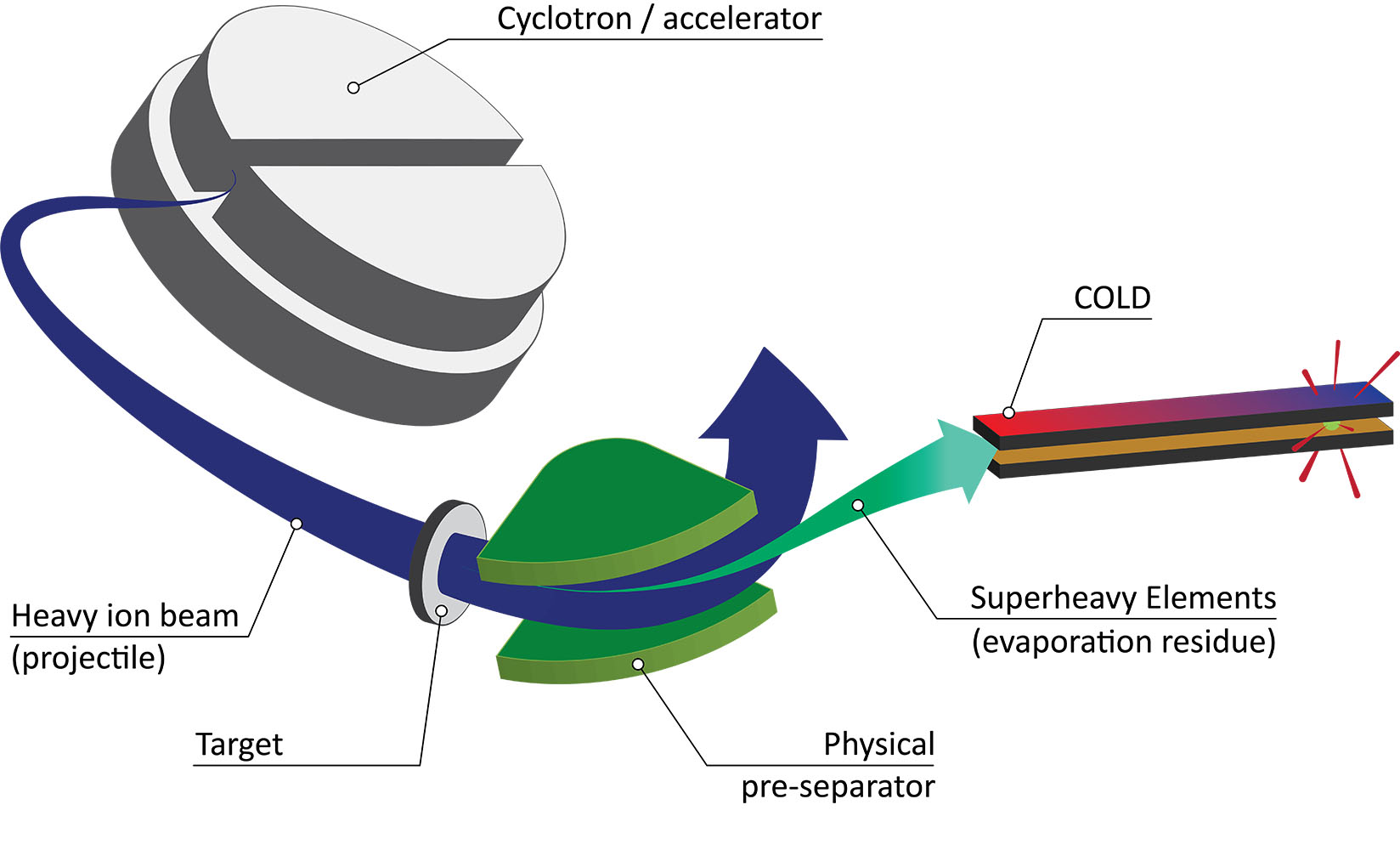

In generating such reaction products, Steinegger collaborates closely with physicists. As a radiochemist, however, his focus is, for example, on the chromatography experiments performed directly afterwards: in a gold-coated channel with a temperature gradient, the newborn elements decay at a certain point with a specific pattern. This is recorded by detectors and finally displayed in a chromatogram. "The decay chain differs depending on the isotope," Steinegger explains, "like a fingerprint. We need this to identify the isotope as well as for further analyses that reveal more about its chemistry."
Generally, the output is low: one atom a day, sometimes one a month. This is due to the low probability of creating superheavy elements. In addition, atoms become more and more unstable as the number of protons increases, which makes detection and analysis more difficult. Furthermore, the experiment must succeed several times to obtain a significant result. Special accelerator facilities are needed for this: "Here at PSI, we use the neutron source SINQ or the proton accelerator to carry out important preliminary experiments and optimize the setting. For the final experiments, however, we need specific large-scale research facilities that are only available in certain places – for instance, in Russia or Japan," Steinegger explains. For this reason, he maintains good contacts worldwide and works on an interdisciplinary basis. That makes radiochemistry particularly exciting.

From relativistic effects to cancer research
The purpose of this research is to learn more about radioactive elements. This makes it possible, for example, to explore relativistic effects, which affect not only superheavy elements, but also heavy elements such as lead or gold. "Relativistic effects give gold its color…or did you know that 80 percent of the voltage of lead-acid batteries, such as those installed in cars, is also due to these effects? That`s why it is important to describe or predict the behavior of such elements well, and here our research results come into play.”
As an assistant professor at the LAC, Patrick Steinegger is also looking forward to introducing students at ETH Zurich to the fascinating world of radiochemistry, ideally with special lectures and practical courses. "Radiochemistry is exciting and good specialists will be needed in the future, for example for the decommissioning of nuclear power plants, for the diagnosis and therapy of cancer through radiopharmacy, or for research like ours." In addition to teaching and research, the scientist also attaches great importance to public relations in the field of radiochemistry.

In the face of all these tasks, Patrick Steinegger recharges his batteries not only in the lab, but also on a friend's farm: "I'm not the vacation type. But when you've mowed a huge field, it's relaxing and you see the output directly. That's not always the case in research." In these situations, it is comforting to look at the expanded periodic table, which is not a rigid relict, but a vivid and tangible proof that shows how much has already been discovered and how much more still awaits discovery.
Further information
external page Youtube-Video Superheavy Elements (Idea: Patrick Steinegger)